Developing a new drug is hard, and it’s risky.
By some estimates, one new drug requires $879 million1 to $2.87 billion2 and 12 to 15 years to develop when accounting for drug candidates that don’t receive regulatory approval.3 To put this into perspective, investigational drugs have a 35% chance of advancing to clinical trials, and only 9 to 14% will progress from Phase I clinical trials to market.2
Given these high costs, companies increasingly prioritize drugs with larger commercial returns, often focusing on treatments for chronic diseases with ongoing demand over short-term therapies or vaccines.
As industry leaders look for ways to mitigate risks and streamline development, AI stands out as a transformative tool. By accelerating research timelines, optimizing manufacturing, and enhancing quality control, AI has the potential to bring more effective and safer therapies to market. Morgan Stanley projects that AI could add 50 new therapies to the market in the next decade, generating over $50 billion in sales. Similarly, McKinsey estimates that generative AI could unlock 2.6% to 4.5% of annual industry revenue, equating to $60 billion to $110 billion.4
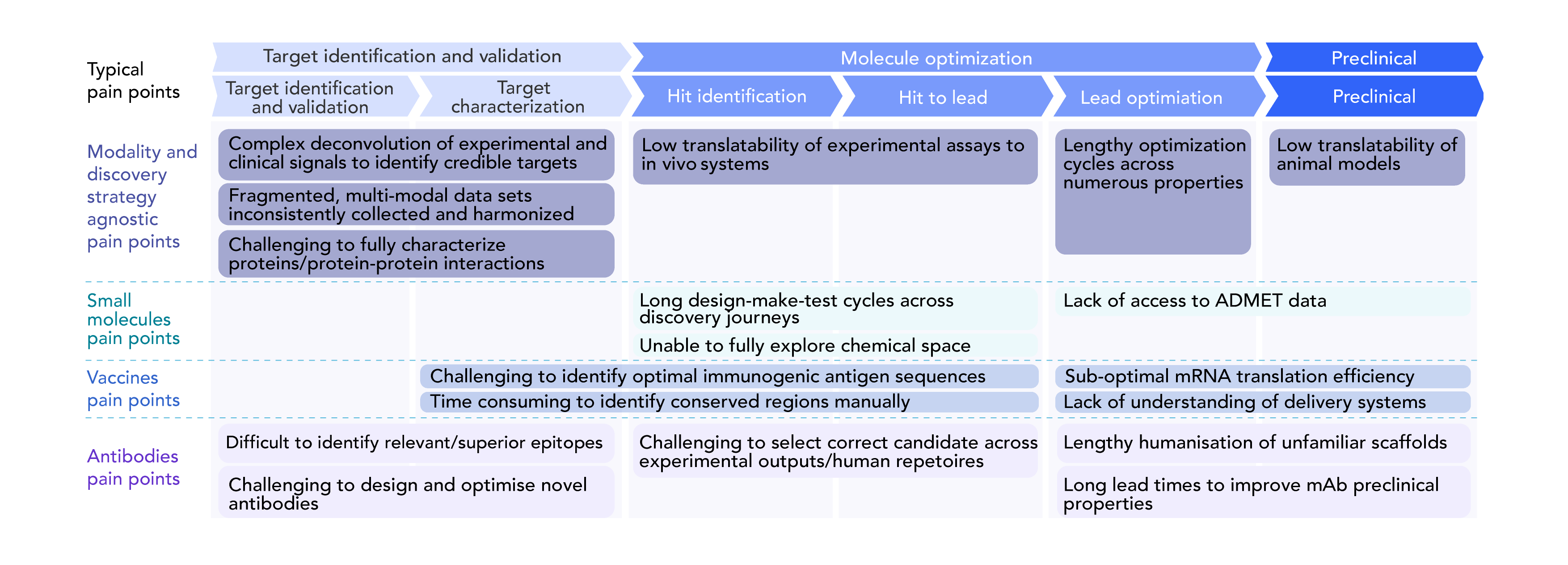
Figure 1. Common pain points in the drug discovery and development process. Pain points are divided between different stages of the drug development process and the type of therapy being designed. Source: Wellcome Trust.3
Current AI applications in large molecule research and optimization
AI is already addressing pain points in biologics discovery and engineering, but the technology has yet to reach its full potential in many areas. Here are three major ways AI is enhancing the drug development process:
- Decreasing time and money required to develop a new drug
- Increasing the probability of regulatory approval
- Creating more novel drugs and targeting more unique targets
For instance, AI can vastly improve the development of all types of vaccines through enhanced epitope selection. Accurately predicting the epitope structures most likely to stimulate a strong immune response and bind well to antibodies and relevant immune cells could significantly reduce the failure rate in vaccine research. AI can also improve the optimization of mRNA vaccine expression and efficacy by enhancing codons and therapy delivery systems.
Moreover, accurate predictions for antibody structures, formats, binding, and other characteristics can streamline biologics development. AI models can also be trained to identify existing or generate entirely new antibody designs that can interact with a specific epitope. Much like small molecules, AI can also be used to tailor antibodies for binding, physicochemical, and humanization properties to optimize the efficacy of the therapy.
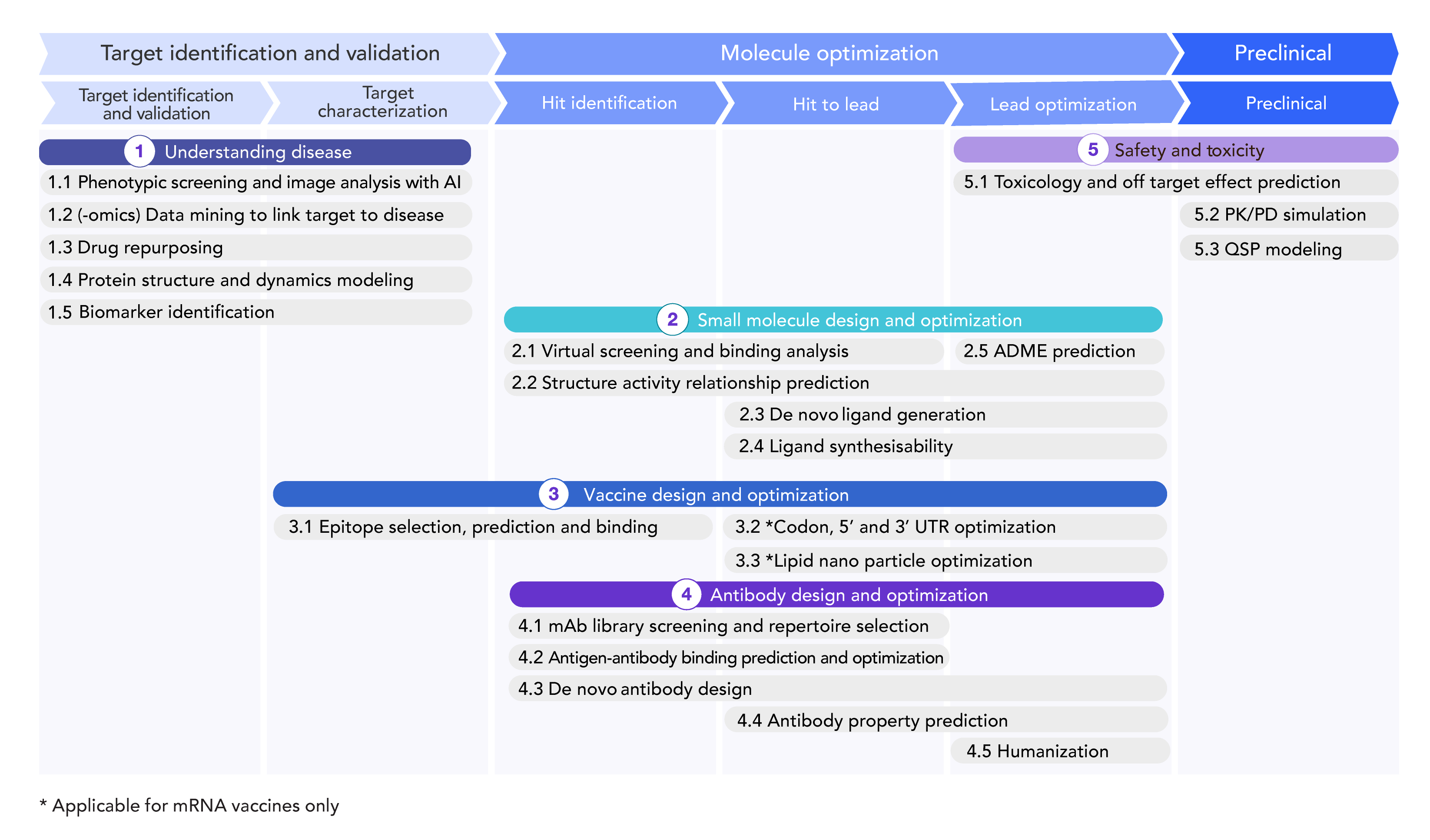
Figure 2. Ways in which AI can improve vaccine and antibody research divided by the stage of research and development. Source: Wellcome Trust.3
Evaluating AI’s impact on large molecule drug discovery
As the biopharma industry seeks measurable gains, executives face the question: How much value can AI truly bring across the biologics discovery and engineering pipeline? Given the novelty of high-powered AI analysis, large amounts of data on AI value in drug discovery don’t exist, but data from larger-scale programs will eventually mature to provide a more accurate metric of AI contributions. This includes 73 AI-derived small molecule, antibody, and vaccine therapies currently in development pipelines,2 15 of which are currently in clinical trials.8
In terms of time and cost savings, AI seems to be living up to the hype (Figure 3). Companies are reporting shorter development timelines and lower costs with AI-driven workflows. Select therapies generated through AI partnerships or AI-derived workflows required an average of four years, rather than five to seven years, before gaining regulatory approval.2 The development of the mRNA SARS-CoV-2 vaccines in the wake of the COVID-19 pandemic also serves as a good example of time savings afforded by AI, albeit on an accelerated track with pandemic use authorizations. The machine learning tool Smart Data Query (SDQ) allowed Pfizer COVID-19 vaccine clinical trial data to be reviewed by the FDA only 22 hours after establishing the primary efficacy case counts.9 According to Pfizer, they estimate this saved the company an entire month before FDA approval by improving clinical trial data quality.9
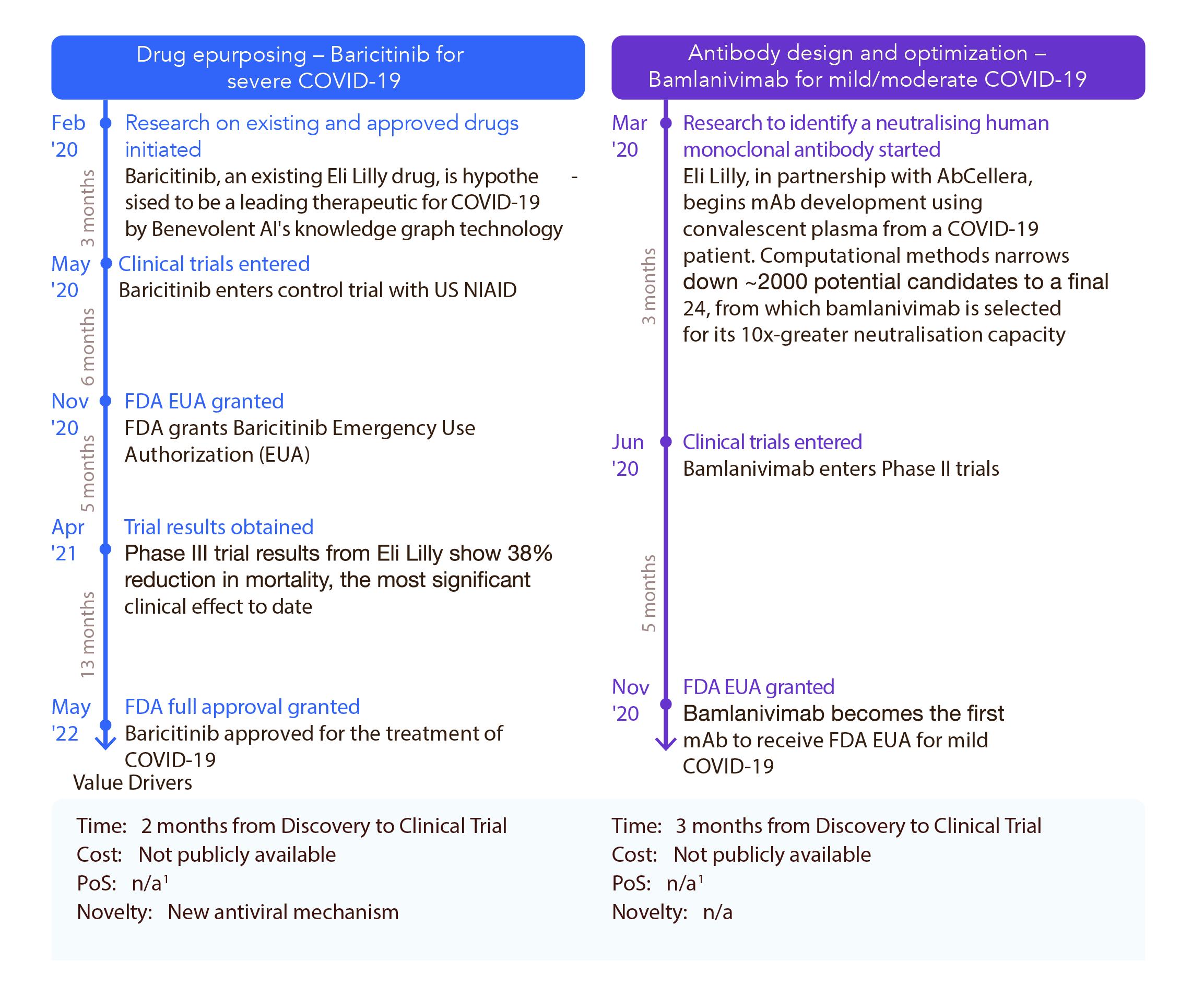
Figure 3. Proof of value example for AI-enabled antibody design and optimization. Source: Wellcome Trust
Barriers to realizing AI’s value in biologics discovery and engineering
Biopharma leaders increasingly recognize AI’s potential to revolutionize the life sciences, including large-molecule and antibody R&D. According to Deloitte, 94% of surveyed business leaders see AI as essential to their success in the coming five years.5 Biopharma companies have already invested over $2.5 billion in AI initiatives,6 with plans to significantly increase spending. For example, Morgan Stanley predicts that AI-related R&D budgets will rise from 1.5% in 2023 to 4% by 2030.7
Despite this growing investment and compelling potential, many companies are still struggling to integrate AI effectively. This gap between the vision for AI and its practical application remains a key hurdle for the industry. Here’s a look at some of the top challenges:
- Data management and quality: Without high-quality, harmonized datasets, AI tools can’t perform effectively. Breaking down data silos between ML and research teams and ensuring accessible, well-structured data remains a primary hurdle.
- Trust in AI tools: Many teams are skeptical of AI’s reliability or struggle to understand how to integrate it effectively within established workflows.
- Limited tool maturity and accessibility: Organizations often find that available AI tools aren’t fully optimized for their specific applications. Fit-for-purpose tools, tailored for unique R&D needs, can bridge this gap.
- Skills and infrastructure: Successful AI adoption requires a skilled workforce and modern infrastructure, which demands both training investments and strategic partnerships with AI specialists.
These barriers must be addressed for organizations to maximize the benefit of AI in biologics discovery and engineering worldwide. Costs and data quality can be two of the most significant specific barriers to AI integration in any biopharma organization.3 The availability of high-quality tools, coupled with a lack of trained personnel and understanding, are common barriers in the pharma industry, leading to intense competition among companies for skilled and specialized employees.3 Importantly, these pain points are addressed specifically by AI bioinformatic companies that specialize in data harmonization, tool development, and workforce training. A summary of specific AI-integration pain points is found in Figure 4.
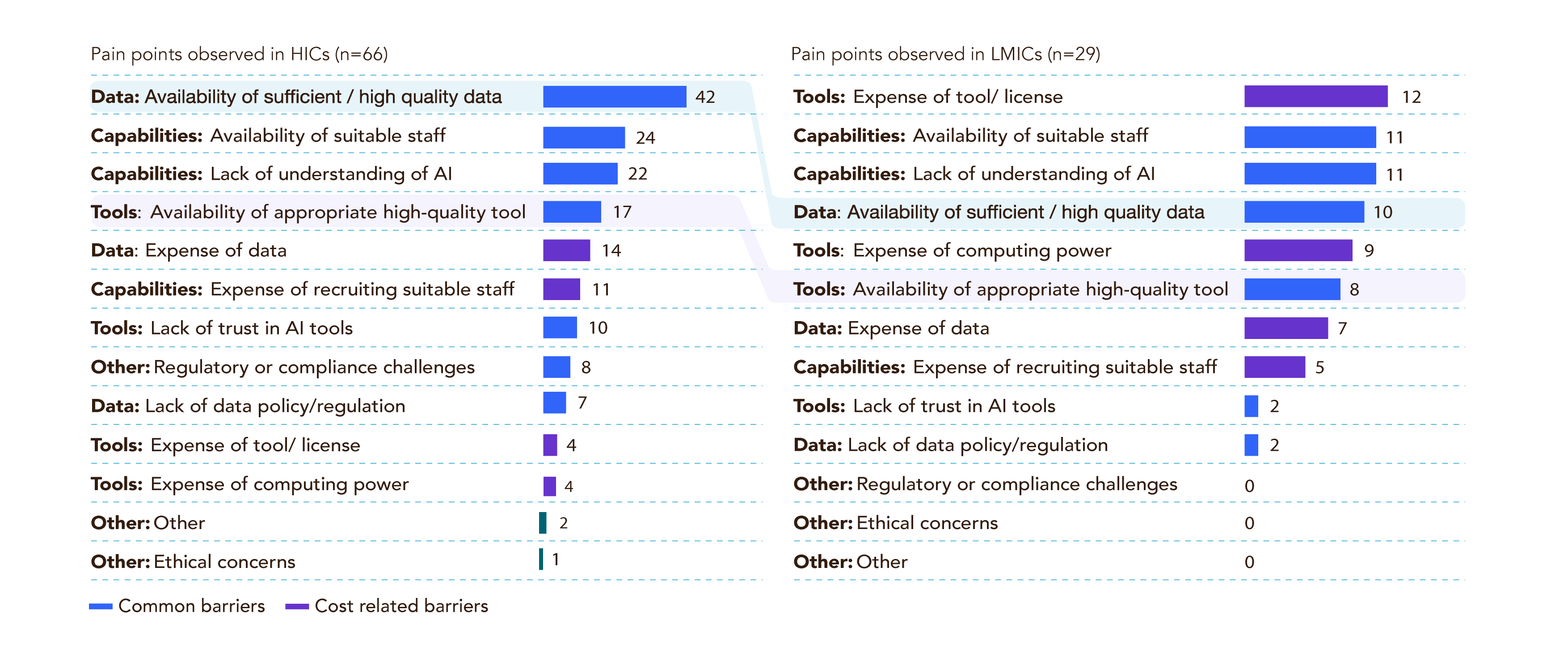
Figure 4. Ranked pain points for high-income (HICs) and low- and middle-income countries (LIMCs) for adopting AI-enabled drug discovery workflows.
Setting the course to AI readiness
Effective integration of AI into drug discovery workflows requires addressing pain points across sectors, locations, and therapies. Solutions designed to reduce barriers, increase and improve datasets, and foster the development of new and better tools will go a long way in reducing barriers to AI adoption.
Biopharma leaders are driving AI initiatives with the goal of accelerating scientific breakthroughs, reducing costs, and delivering better therapeutics. However, many companies face challenges at the early stages of AI integration, constrained by legacy systems and fragmented data. To bridge this gap, organizations must rethink their data strategies and commit to creating AI-ready environments. This means transforming raw scientific data into harmonized, scalable, and compliant formats, supported by an open, collaborative data architecture built specifically for AI.
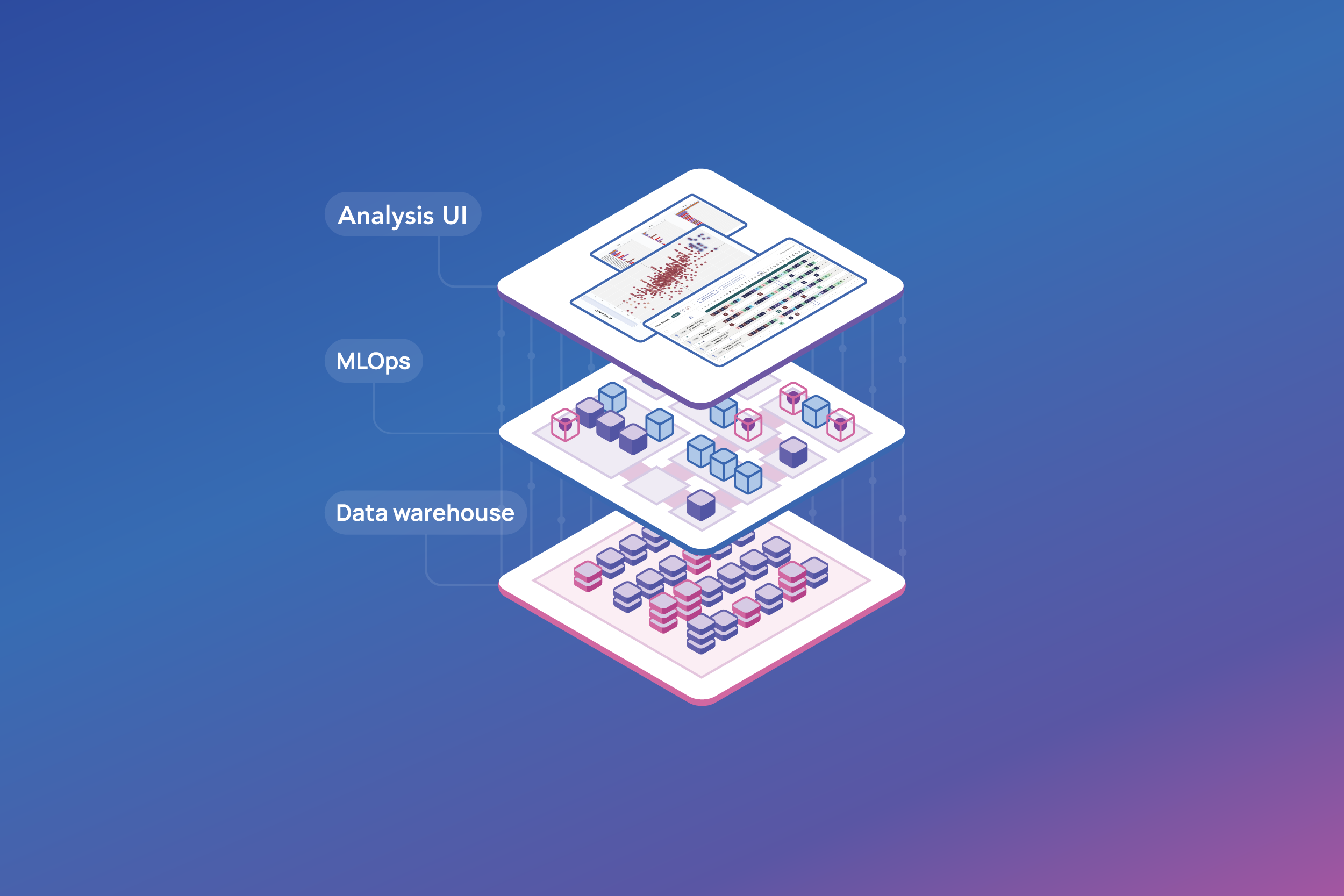
ENPICOM offers a comprehensive solution to support this journey:
- Robust data foundation. Without structured data, AI tools cannot perform effectively. ENPICOM’s scalable data foundation provides fast, secure access to harmonized data, integrating seamlessly with existing storage or data warehouse systems to eliminate data silos.
- Analysis pipeline automation and consolidation. Process automation enhances data harmonization and minimizes human error, resulting in reduced development risks and maintenance costs. With a comprehensive set of API and integration tools, the ENPICOM Platform ensures smooth data flow and compatibility with your research ecosystem, including specific data environments, LIMS, bioinformatics pipelines, and proprietary ML models. This streamlined approach reduces complexity and fosters collaboration, ensuring data integrity across all stages of biologics discovery.
- AI integration. Unified, fully integrated platforms enhance AI efficiency by harmonizing legacy and newly generated data, optimizing large-scale data accessibility, and providing comprehensive AI lifecycle-management tools. A robust MLOps framework ensures quick deployment of ML models along with the required tracability and provenance information. In addition, the ENPICOM Platform provides a fully integrated infrastructure that allows ML scientists to deploy models directly onto the platform. Lab scientists can easy access and run AI models for analysis and decision-making, fostering innovation and accelerating breakthroughs.
We are at a pivotal moment where AI can transform the future of drug discovery. By optimizing data for AI-driven workflows, your organization can unlock new possibilities in therapeutic development. Reach out to our experts to explore how AI can enhance your biologics discovery and engineering efforts.
References
- Solomon, L. (July 2, 2024.) Mean cost of bringing new drug to U.S. market is $879.3 million. Drugs.com. Accessed September 26, 2024.
- DiMasi, JA, et al. (May 2016). Innovation in the pharmaceutical industry: New estimates of R&D costs. J Health Econ. Accessed October 10, 2024.
- Understanding the potential of AI in drug discovery: Current status, barriers and future opportunities. (June 25, 2023.) Wellcome Trust. Accessed September 26, 2024.
- Chui, M et al. (June 2023.) The economic potential of generative AI: The next productivity frontier. McKinsey & Co. Accessed October 24, 2024..
- Fueling the AI transformation: Four key actions powering widespread value from AI, right now. (October 2022). Deloitte’s State of AI in the Enterprise, 5th Edition report. Accessed October 24, 2024.
- Chadha, S et al. (2023.) Technology Vision 2023 for Biopharma: Merging atoms and bits. Accenture. Accessed October 24, 2024.
- Why Artificial Intelligence Could Speed Drug Discovery. (September 9, 2022.) Morgan Stanley. Accessed October 24, 2024.
- Mallatt, S. (March 4, 2024.) Expert insights: How artificial intelligence is transforming drug development. Alphasense. Accessed September 26, 2024.
- mRNA and artificial intelligence for advanced vaccine innovation. Pfizer. Accessed October 10, 2024.