Recombinant monoclonal antibodies (mAbs) have become increasingly used as therapeutics for various medical conditions. Over the last four decades, more than 100 mAbs have been approved by the United States Food and Drug Administration (US FDA) [1]. Moreover, a multitude of mAbs and alternative antibody scaffolds, including full-length mAbs, bi-specific mAbs, antibody fragments, and drug-antibody conjugates, are currently being evaluated in phase III studies or are under review for approval [2]. These novel therapeutics bind to clinically significant molecular targets in hematology, oncology, infectious diseases, autoimmune diseases, and cardiovascular diseases, enabling therapies for previously undruggable diseases.
Antibody discovery is the identification and isolation of antibodies with high therapeutic potential. It typically starts with the research and identification of molecular targets that have high therapeutic potential. These targets are molecules, often proteins, involved in the molecular mechanisms underlying a specific disease. Such molecules, or their parts, can be introduced to animal models to induce an immune response resulting in generation of antibodies against the target of clinical interest. Antibodies with high therapeutic potential from animals can be obtained using hybridoma or single cell technologies. The antibody repertoires retrieved from animals can also be used in creation of immune or naive libraries to be used in display discovery campaigns. In this blog, we discuss the diverse animal models used in antibody discovery and their roles in the research process.
Animal models in antibody discovery
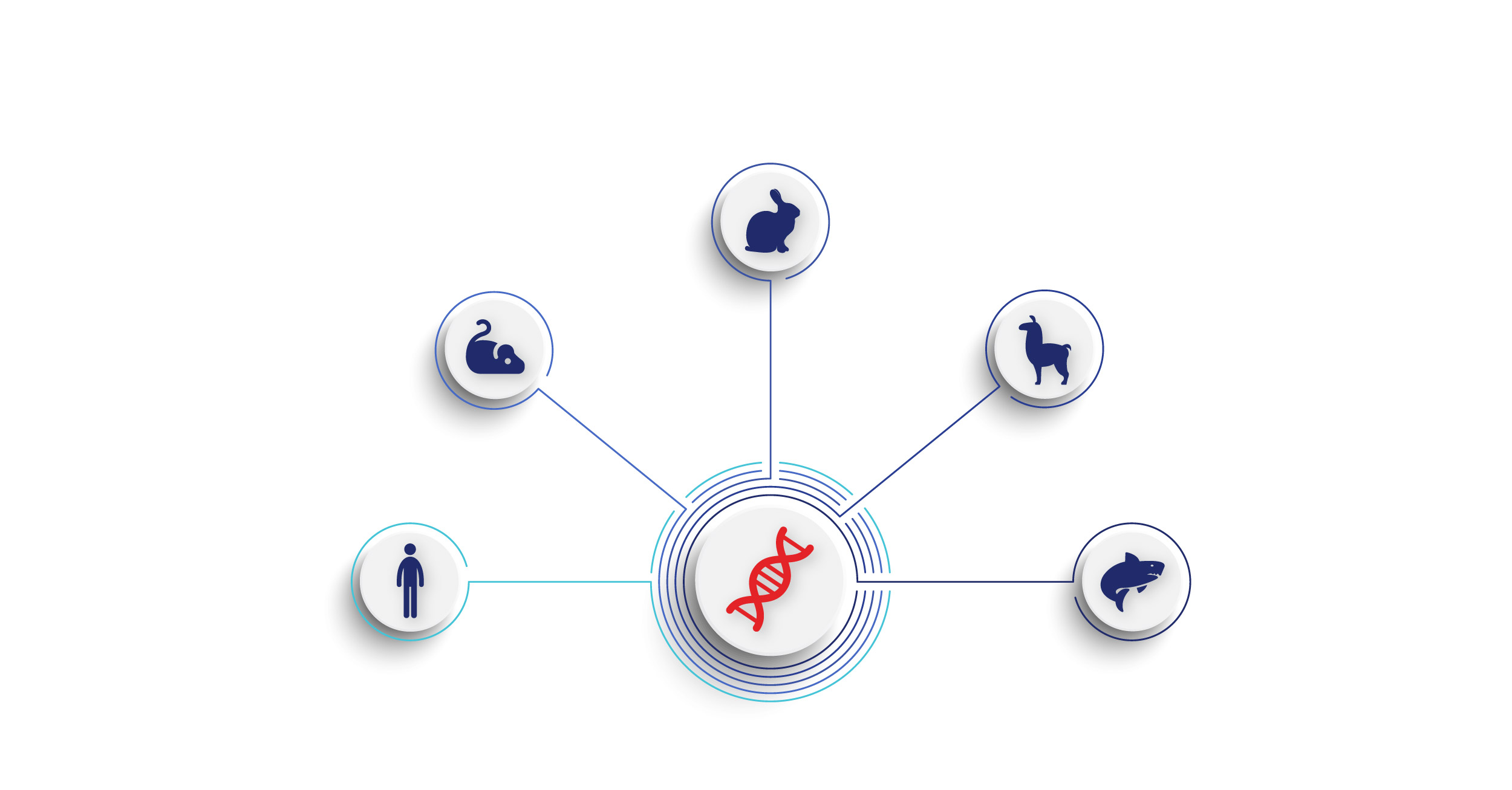
Animal models are widely used for the discovery of antibodies for therapeutic, diagnostic, and research purposes. The use of animals for therapeutic antibody discovery has been proven to be successful through the decades. In fact, the majority of US FDA-approved antibody-based therapeutics have been discovered using (transgenic) animals [3]. Alongside the reliability, using animals in the discovery process has the advantage of retaining natural maturation of the antibodies. Various animals have been used for antibody discovery to research antibodies with different structures, shapes, and sizes for their therapeutic application potential. The traditional animal models used in therapeutic antibody discovery are mice, rats, and rabbits used in generation of IgGs. These are generally inexpensive, versatile, and well-established animal models in research laboratories.
In most applications these can provide sufficiently diverse antibodies to further develop into therapeutics. However, animals such as chickens, camelids (e.g., alpacas, llamas, and camels), and nurse sharks have become increasingly interesting due to the distinct nature of their antibodies. Camelids and nurse sharks produce heavy chain only antibodies (HCABs) that can be made into smaller therapeutics that can access targets that IgGs do not. Nanobodies from camelids (VHHs) and variable new antigen receptors (VNARs) from nurse sharks are such examples of therapeutic antibody fragments discovered from these animals. Chickens produce IgY antibodies, which can have better binding and developability properties compared to their IgG counterparts. Other animals including hamsters, cows, guinea pigs, ferrets, geese, pigs, alligators, and horses are also used in antibody discovery but far less often. The antibody diversity of the animal kingdom provides us with a treasure trove of new potential therapeutics to discover. Animal models play an important role in characterizing the therapeutic candidates as well. Therapeutic potential and toxicity of mAb are generally studied in non-human primates such as cynomolgus and rhesus macaques because these animals are the closest to humans immunologically and physiologically [4].
Antibodies generated in animals typically induce an immune response when administered directly to humans due to their foreign origin. The immunogenicity of the antibody can be mitigated by a humanization process. This can be achieved by introducing mutations that alter the antibody sequence to better resemble those found in humans, or by grafting the binding regions of the animal-derived antibody into a human antibody scaffold. However, humanizing a sequence has the risk of reducing the binding and functionality of the antibody, and assessing the humanness can help select candidates that will need less modifications to become valid therapeutics. The risk and additional cost that comes with humanization can be also circumvented using transgenic animals genetically engineered to contain human immunoglobulin loci. These transgenic animal models provide a reliable platform for immunization-based antibody discovery that produces fully human antibodies [3].
Although animals provide many benefits to antibody discovery such as in vivo maturation, there are various drawbacks to working with them other than the humanization process. First, immunization studies are time-consuming, and usually require multiple injections over the course of various weeks/months to induce an adequate antibody response. It is also important that immunization antigens must be soluble and accessible which may require modifications to the original target. This may introduce additional risks such as loss of natural target conformation or increase costs of the discovery process. Furthermore, these antigens may lose structural integrity when injected in vivo or when combined with adjuvants needed to increase vaccine immunogenicity. Additionally, there is limited to no control over the antibody response during immunization studies. As a result, the antibody response can be skewed towards the immunodominant regions which may restrict the epitope specificity against an antigen. There are mitigation strategies to some of these drawbacks (e.g., modification-free membrane stabilization, epitope sorting), but they should still be considered when weighing the pros and cons. Finally, there are ethical considerations about continuing the widespread use of animals for antibody discovery while animal-free options like display technologies have been shown to be promising alternatives [5].
Analysis of antibody repertoires after immunization
The immunization of animals has proven to be an efficient approach to generate a wide array of antigen-specific antibodies, but low isolation throughput and characterization limited the discovery. The development of high-throughput sequencing technologies enabled to interrogate the immune repertoire to an unprecedented level of depth. This allows to harness the full potential of in vivo maturation by capturing many more antibodies elicited after immunization. In this way, clones isolated with hybridoma or single B-cell isolation approaches can now be used to mine NGS repertoires and find alternative (related) candidates with better characteristics. High-throughput sequencing technologies, however, generate orders of magnitude larger volumes of data, requiring more computational power and expertise to analyze. At ENPICOM, we have developed the IGX Platform to remove the complexity of managing and analyzing immune repertoire sequencing data. Sequencing technology-agnostic and code-free, it enables every scientist to focus on discovering the best antibodies by simplifying complex analysis like clustering or computing phylogenetic trees. Additionally, it allows you to predict exposed liabilities in your candidates and select the candidates with more chances to become life-saving therapeutic treatments. Contact us and find out how the IGX Platform can help you find the best antibody from your immunization studies.
References
[1] Antibody therapeutics approved or in regulatory review in the EU or US – The Antibody Society. https://www.antibodysociety.org/resources/approved-antibodies/.
[2] Kaplon, H., Chenoweth, A., Crescioli, S. & Reichert, J. M. Antibodies to watch in 2022. MAbs 14, 2014296 (2022).
[3] Lu, R.-M. et al. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 27, 1 (2020).
[4] Cauvin, A. J., Peters, C. & Brennan, F. Chapter 19 – Advantages and Limitations of Commonly Used Nonhuman Primate Species in Research and Development of Biopharmaceuticals. in (eds. Bluemel, J., Korte, S., Schenck, E. & Weinbauer, G. F. B. T.-T. N. P. in N. D. D. and S. A.) 379–395 (Academic Press, 2015). doi:https://doi.org/10.1016/B978-0-12-417144-2.00019-6.
[5] Laustsen, A. H., Greiff, V., Karatt-Vellatt, A., Muyldermans, S. & Jenkins, T. P. Animal Immunization, in Vitro Display Technologies, and Machine Learning for Antibody Discovery. Trends Biotechnol. 39, 1263–1273 (2021).